What are the C.I.P. (Clean-in-Place) Best Practices for Your Process?
CIP can save time, materials and labor, which ultimately means money. Yet, while CIP has been around for a long time, it is much more prevalent outside of the US than in, which leaves a lack of experience and knowledge to go on, and it is not an exact science. Not even the Food and Safety Modernization Act (FSMA) contains any validation requirements for Clean-In-Place (CIP) procedures. This isn’t helping US companies as they look to implement CIP into their operations.
Here at Springer Pumps, our customers are showing a greater interest in CIP. While at the same time we are also seeing a bigger product focus on CIP from our vendors. While having both the interest and the products to support it are good, there are some hurdles to overcome when it comes to implementing CIP systems. The biggest of these is that there are no strict guidelines for CIP processes, not just in the US, but abroad.
So when customers ask us what the CIP procedures are for a given pump the answer is: It depends.
Broadly Outlined, a CIP Process Should Contain the Following Steps:
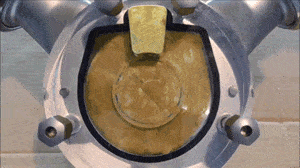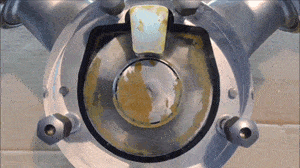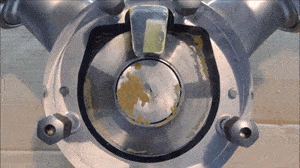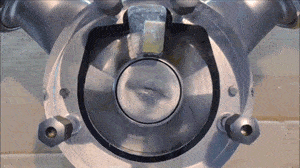
- Run a flush with clear water at full pump speed, with no back pressure, to clean out the majority of the process fluid.
- Run another flush with the cleaning fluid reaching a minimum velocity of 1.5 m/sec (5 ft/sec) and a differential pressure between the inlet and outlet of the pump of at least 2 bar / 29 psi.
- If necessary, run another flush using the sanitation fluid for the amount of time necessary to ensure proper system sanitization.
- Perform swab tests for high sanitation applications. Especially those with potential allergen issues.
The steps outlined above take TIME, and the time it takes depends on several factors, including:
- Pump(s) used
- Line sizes (inlet and discharge)
- Process fluid/media/soil
- Cleaning/Sanitation chemicals used
- The temperature of the flush water
All of these are considered in light of the most important one: What is the end goal? Is it just a clean system, a sanitary system, or a system free from all contaminants including allergens?
Taken all together, these will give you an idea of what the basic parameters are for CIPing a given system. But that idea must be proven out through trials in actual production and refined from there.
While we cannot tell you what exactly it will take to CIP your systems and components, we will work with you to provide the correct type and size pumps to get the job done.
OpX Leadership Network has put together a detailed document on CIP processes and what is required for them, along with a video presentation they made from PACK EXPO with Caitlin Lucia, Senior Process Engineer at Campbell Soup Company. Some of the content for this article is from their paper and is used with permission.
You can schedule a call or meeting with us if you’re considering Clean-in-Place and need process pumps that are designed FOR CIP or a highly efficient Direct Contact Water Heating System.